 |
|
|
 |
|
|
 |
|
|
|
|
|
|
Matter, and Physical and Chemical Changes
Asa Packer Elementary
School, Spring 2009
Grade 1 |
|
|
|
|
| |
|
Dr. DeLeo is a teacher from Lehigh University, where they teach big kids. He turned one of the rooms in our school into a "Science Lab." There were all sorts of cool things all around the room! We hoped that we would get to do something with every one of them!
Dr. DeLeo told us that he started running science programs at schools a long time ago, right here at Asa Packer. When he started, his daughter was in third grade here, and now she has been out of college for six years and is about to get married! |
|
|
|
|
|
| |
|
|
|
In this program, we learned what matter is made of and how it can change. We all knew that matter could be solid (like our desks), liquid (like milk), or gas (like the air). And, we even knew that water could be solid, liquid, or gas, depending on how hot or cold it is. If the Earth were too far from the Sun, all of the water on the Earth would be frozen into ice, like in our freezers. If the Earth were too close to the Sun, all of the water would boil and turn to steam, a gas. It’s a good thing that the Earth is just the right distance from the Sun so that most of the Earth’s water is liquid (though some of it is ice at the North and South poles).
|
|
|
|
| |
|
When water changes from solid to liquid or from liquid to gas, it is called a physical change. We learned that matter is made up of tiny objects called atoms. Atoms are so small that it took scientists thousands of years to even figure out that they existed. When the temperature is lower, the atoms move more slowly. So in a solid, such as ice, the atoms move slowly and stick tightly together. If you take an ice cube out of the freezer and let it warm up, the atoms begin to move more quickly. They are still close together, but now they can move enough to roll over one another, and we have a liquid. So, when you jump into a swimming pool (in the summer!), the water moves out of your way. Also, it can be poured and it takes the shape of the container you pour it into. If you place the water in a pan on the stove and make it real hot, the atoms become unstuck and zip all around the kitchen. Now, you have steam, which is a gas.
|
|
|
|
|
|
|
|
| |
|
|
|
Dr. DeLeo brought in a special machine that could make little metal balls bounce around between two pieces of glass. When he turned the machine to "high," the balls bounced all over the little box, like atoms moving in a gas. We could see them moving on a television screen because Dr. DeLeo aimed a TV camera at them. When Dr. DeLeo turned the machine off so the little balls didn't move, they all settled tightly together on the bottom. This is like a solid. When he turned the machine to low, so they only shook a little, they were close together like in a solid, but they could roll over one another. Dr. DeLeo showed us how the balls would then pour like a liquid.
|
|
|
|
| |
He showed us a solid called silicon. Even though this is one of the most common elements on Earth, you would never find it like this on the ground. When purified, it is used to make our electrical things work, and it can even turn light into electricity (solar cell running a motor on the far right). |
|
|
|
|
|
|
|
|
Atoms are held together by electric forces. These are the same forces that hold a balloon on the wall. Sometimes we call this static electricity. Dr. DeLeo used a clear rod that was rubbed against a piece of cloth to pull an aluminum soda can across the floor without even touching it. |
|
|
|
| |
|
|
|
We learned that the air contains oxygen and nitrogen. We need oxygen to breathe. The air is mostly nitrogen. If we make nitrogen very cold, the atoms are pulled closer together by the electric forces, and it becomes a liquid. Liquid nitrogen is often used to make things very cold. If you dip a rubber band into liquid nitrogen, it becomes hard and brittle. We all got to dip rubber bands into liquid nitrogen, after we put on safety goggles and learned how to be careful. Liquid nitrogen is 300 degrees below zero! Here are some pictures of us dipping our rubber bands in liquid nitrogen and breaking them.
|
|
|
|
|
|
|
Since Rocks are interesting solids that form in nature, Dr. DeLeo brought in rocks, and even fossils, for us to look at under special magnifying glasses. We love rocks and fossils! |
|
|
|
|
|
|
|
|
|
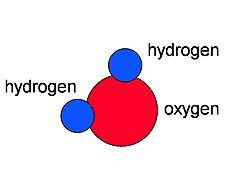 Atoms can be put together in many different ways. When a few atoms are connected together, we call this a molecule. When we put hydrogen and oxygen together, we get water. A water molecule has two hydrogen atoms and one oxygen atom. It kind of looks like Mickey Mouse. We discovered that a little bit of static electricity will bend a stream of water. We can use stronger electric forces by putting electricity into the water to pull the water molecules apart. Atoms can be put together in many different ways. When a few atoms are connected together, we call this a molecule. When we put hydrogen and oxygen together, we get water. A water molecule has two hydrogen atoms and one oxygen atom. It kind of looks like Mickey Mouse. We discovered that a little bit of static electricity will bend a stream of water. We can use stronger electric forces by putting electricity into the water to pull the water molecules apart.
Before we did this, Dr. DeLeo talked to us about safety issues. He said that electricity and water are a dangerous combination. We should never take something that's plugged-in into water with us. We could get killed. Dr. DeLeo put electricity into water in a very controlled way, and we helped him by watching to make sure that his hands weren’t by the water when the electricity was turned on.
|
|
|
|
|
|
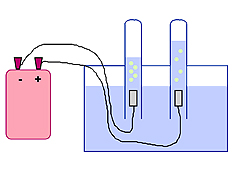 When the electricity was on, bubbles rose in the water filled test tubes. Dr. DeLeo told us that this wasn’t the water boiling. The bubbles going into one tube were hydrogen, which is a gas. The bubbles going into the other tube were oxygen, which is also a gas. Dr. DeLeo had us ask him to prove that the gases were hydrogen and oxygen. He proved that the one tube contained hydrogen by holding a match by it. It went “POP!!" as the hydrogen exploded. The oxygen was trickier since it doesn’t burn. However, things don’t burn without it. That is why we “stop, drop, and roll” if we catch on fire – to smother the fire by cutting off its oxygen. Dr. DeLeo lit a thin piece of wood on fire, and then blew it out so it was only glowing red. When he placed the glowing wood into the pure oxygen in the test tube, it burst back into flames, over and over again. In pure oxygen, things burn better. When the electricity was on, bubbles rose in the water filled test tubes. Dr. DeLeo told us that this wasn’t the water boiling. The bubbles going into one tube were hydrogen, which is a gas. The bubbles going into the other tube were oxygen, which is also a gas. Dr. DeLeo had us ask him to prove that the gases were hydrogen and oxygen. He proved that the one tube contained hydrogen by holding a match by it. It went “POP!!" as the hydrogen exploded. The oxygen was trickier since it doesn’t burn. However, things don’t burn without it. That is why we “stop, drop, and roll” if we catch on fire – to smother the fire by cutting off its oxygen. Dr. DeLeo lit a thin piece of wood on fire, and then blew it out so it was only glowing red. When he placed the glowing wood into the pure oxygen in the test tube, it burst back into flames, over and over again. In pure oxygen, things burn better.
|
|
|
|
| |
|
|
|
|
| On the far left is a picture of the machine that Dr. DeLeo used to make the hydrogen and oxygen. Click the play button on the picture on the left to play a video. Dr. DeLeo told us that even though it was only a little "pop," he should have been wearing goggles. Sometimes, even grown-ups forget, and kids need to remind them! |
|
|
|
| |
|
We got to ask Dr. DeLeo a lot of questions. He said we were very smart, and thought that maybe we were getting some kind of special brain vitamins. But we think we're naturally smart! And, we have great teachers! Dr. DeLeo gave us each a gift: a magnifier in a special pouch. This was a really fun day!
|
|
|
|
|
|
|
|
|
I hope you have enjoyed this web presentation as much as we enjoyed sharing the actual learning experience with your son or daughter. Although we have endeavored to exclude photographs where permission has been denied, it is possible for errors to occur. If you would like us to remove a photograph of your son or daughter for any reason, please send me an e-mail message at lgd0@lehigh.edu or call me at 610-758-3413, and we will remove it promptly. Please note that we will never associate a child's full or last name with a photograph except in circumstances where special permission was explicitly provided. Thank you. Gary DeLeo. |
|
|
|
|
|
|
|
|
|